NADT-work
Mission
NADT-work, an abbreviation for Nucleic Acid Diagnostics and Therapeutics Network, is a non-profit research consortium comprised of chemists, biophysicists, molecular biologists, and clinicians with a common interest in the exploration and development of nucleic acid-based molecular tools and technologies for the detection and treatment of genetic diseases. The mission of NADT-work is to accelerate the pace of research discovery and clinical translation, with the overarching goal of improving human health, through collaborative research and innovative approach in molecular design, chemical synthesis, and translational development.
Technology
Built on work of Nielsen, Egholm, Berg, and Buchardt, in the development of peptide nucleic acid (PNA), first reported in 1991 (Science 1991, 254, 1497), the chemistry team at Carnegie Mellon University has tweaked the chemical structure of PNA by installing a stereocenter at the gamma backbone—hence the name γPNA. This backbone stereochemical modification transforms PNA oligomer from a random-fold into a right-handed (RH) or left-handed (LH) helical motif depending on the stereochemistry, and enables the conformationally-matched RH-γPNA to hybridize to DNA or RNA strand with high affinity and sequence specificity and invade double helical B-form DNA (B-DNA) without sequence restriction. Further improvements in water solubility and biocompatibility were made by incorporating diethylene glycol moiety in the sidechain, and in cell permeability by conjugating the guanidinium group. γPNA is synthetically versatile, in that its chemical structure can be readily modified and its physical and pharmacological properties can be fine-tuned. This synthetic flexibility, coupled with its superior hybridization properties and recognition orthogonality, makes γPNA an attractive nucleic acid platform for diagnostics, therapeutics, and molecular engineering.
Material availability
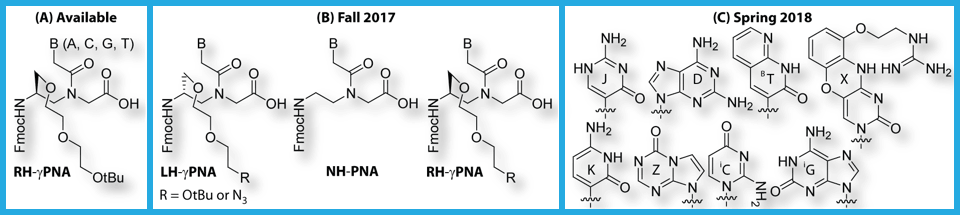
The latest γPNA version (A) is available to collaborators upon request. The next round of chemical building blocks, including LH, NH (nonhelical), and RH conformers (B), with diethylene glycol sidechain as well as that with terminal azido group, is expected to roll out in fall of 2017. Inclusion of the azido group will allow further chemical modifications on-resin, including enabling click reaction and incorporation of other chemical functionalities, such as phosphate, amine, guanidine, piperazine, and so forth. Chemical modifications in the backbone will be augmented by nucleobase derivatives with different binding strengths and recognition orthogonality (C), permitting selective fine-tuning of binding affinity and specificity. These building blocks are expected to come online in spring of 2018.
Relevant publications
Conformational preorganization (JACS 2006, 128, 10258; JACS 2010, 132, 10717), solubility/biocompatibility (JOC 2011, 76, 5614), cellar uptake (JACS 2003, 125, 6878; JACS 2006, 128, 16104; JOC2009, 74, 1509; ACS Chem. Biol. 2013, 8, 345), DNA invasion (JACS 2007, 129, 15596; ChemBioChem. 2008, 9, 2388; JACS 2009, 131, 12088; Biochemistry 2011, 50, 3913; ChemBioChem. 2012, 13, 56), recognition orthogonality (JACS 2015, 137, 8603), cyclotide (JACS 2012, 134, 4041), electronic bar coding (Nano Lett. 2012, 12, 1722), gene correction (Nature Commun. 2016, doi:10.1038/ncomms13304).